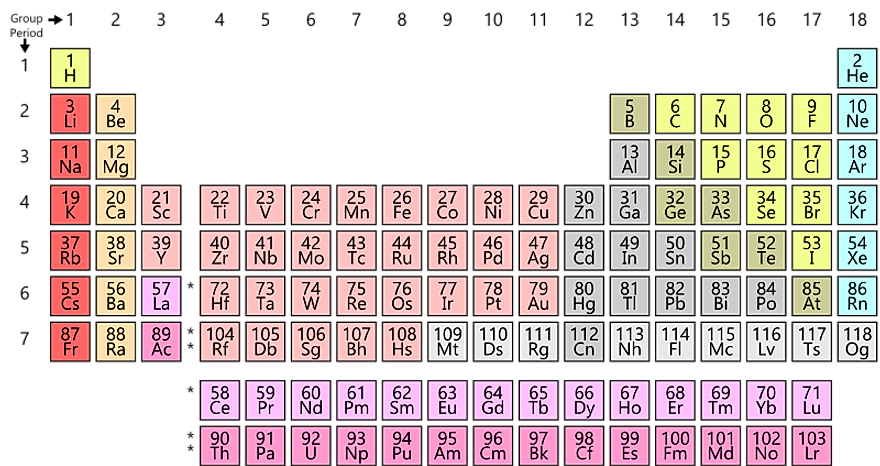
- There are 118 parts on periodic desk
- The variety of protons in a component provides the atomic variety of the component
- In 2016 4 extra parts had been added into the periodic desk
A component refers to a substance manufactured from atoms of the identical type. All of the atoms in a selected component bear the identical atomic quantity. Components can’t be damaged additional into smaller substances utilizing chemical reactions. Nevertheless, they’ll solely be reworked into different parts by nuclear procedures. The atoms in a component comprise the identical variety of protons, however the variety of neutrons varies. When the variety of protons in an atom is modified, then the component modifications.
Properties of Components
Each recognized component has a reputation and a quantity, that are listed within the periodic desk. The periodic desk outlines every component’s electron configuration, the atomic variety of the component, and the chemical properties of the component. The atomic quantity refers back to the variety of protons discovered within the atom of a component. Components will be categorized into three main teams that embrace metals, nonmetals, and metalloids. The weather discovered on the left aspect of the periodic desk are sometimes metals. Whereas the weather on the appropriate aspect of the periodic desk are non-metals. Some parts like hydrogen and sodium are standard whereas others like dysprosium stay unknown as a result of they’re not often used. Components like copper, carbon, and silver have been in existence for hundreds of years.
Present Variety of Components
The periodic desk accommodates a complete of 118 parts. 4 of those had been included on the listing in 2016. These are Nihonium (113), Moskovi (115), Tennessine (117), and Oganesson (118). The primary 98 parts listed within the periodic desk happen naturally whereas the remaining can solely be present in nuclear accelerators and laboratories. Thirty-two of the 98 parts are of their pure kind. The remainder exist as compounds. Eighty of the pure parts are steady, which means that they can’t be subjected to radioactive decay. Ten of the 98 parts solely exist in hint quantities. Sometimes, all the weather of the periodic desk with a better atomic quantity than lead are unstable, thus topic to radioactive decay. Though a number of of the found parts exist naturally, only some of those exist of their native kind. Among the many few are noble gases that don’t kind compounds simply, in addition to metals like copper, silver, and gold. Non-metals that fall into this class embrace nitrogen, oxygen, and carbon. Components that don’t exist of their native kind embrace alkali and alkaline metals in addition to uncommon earth parts.
Uncommon vs Native Components
Uncommon parts are obtained via the radioactive decay of some frequent parts. As an illustration, francium outcomes from decayed actinium. Quite a few the weather listed within the periodic desk just lately could have been produced via the decay of unknown parts which were in existence for a very long time. Native parts, alternatively, are naturally occurring parts in an uncombined kind. Nevertheless, only some native parts are present in compound kind.
The Way forward for the Periodic Desk
Six new parts had been found between 2012 and 2016, filling the gaps that had been remaining on the backside of the periodic desk. The 12 months 2019 marked the a hundred and fiftieth 12 months for the reason that desk was established. Because the chemical properties of recognized parts proceed to alter, new discoveries of parts proceed to happen. Many of the periodic desk modifications will outcome from human-made parts made by scientists utilizing excessive vitality accelerators. Nevertheless, in contrast to pure parts that may be dealt with, these artificial parts are more likely to be unstable, thus decaying shortly. Nonetheless, there’s a chance for extra thrilling discoveries within the atomic world.
How Many Components Are There?
| Rank | Image | Origin of title[2][3] | |||
|---|---|---|---|---|---|
| 1 | H | Hydrogen | |||
| 2 | He | Helium | |||
| 3 | Li | Lithium | |||
| 4 | Be | Beryllium | |||
| 5 | B | Boron | |||
| 6 | C | Carbon | |||
| 7 | N | Nitrogen | |||
| 8 | O | Oxygen | |||
| 9 | F | Fluorine | |||
| 10 | Ne | Neon | |||
| 11 | Na | Sodium | |||
| 12 | Mg | Magnesium | |||
| 13 | Al | Aluminium | |||
| 14 | Si | Silicon | |||
| 15 | P | Phosphorus | |||
| 16 | S | Sulfur | |||
| 17 | Cl | Chlorine | |||
| 18 | Ar | Argon | |||
| 19 | Ok | Potassium | |||
| 20 | Ca | Calcium | |||
| 21 | Sc | Scandium | |||
| 22 | Ti | Titanium | |||
| 23 | V | Vanadium | |||
| 24 | Cr | Chromium | |||
| 25 | Mn | Manganese | |||
| 26 | Fe | Iron | |||
| 27 | Co | Cobalt | |||
| 28 | Ni | Nickel | |||
| 29 | Cu | Copper | |||
| 30 | Zn | Zinc | |||
| 31 | Ga | Gallium | |||
| 32 | Ge | Germanium | |||
| 33 | As | Arsenic | |||
| 34 | Se | Selenium | |||
| 35 | Br | Bromine | |||
| 36 | Kr | Krypton | |||
| 37 | Rb | Rubidium | |||
| 38 | Sr | Strontium | |||
| 39 | Y | Yttrium | |||
| 40 | Zr | Zirconium | |||
| 41 | Nb | Niobium | |||
| 42 | Mo | Molybdenum | |||
| 43 | Tc | Technetium | |||
| 44 | Ru | Ruthenium | |||
| 45 | Rh | Rhodium | |||
| 46 | Pd | Palladium | |||
| 47 | Ag | Silver | |||
| 48 | Cd | Cadmium | |||
| 49 | In | Indium | |||
| 50 | Sn | Tin | |||
| 51 | Sb | Antimony | |||
| 52 | Te | Tellurium | |||
| 53 | I | Iodine | |||
| 54 | Xe | Xenon | |||
| 55 | Cs | Caesium | |||
| 56 | Ba | Barium | |||
| 57 | La | Lanthanum | |||
| 58 | Ce | Cerium | |||
| 59 | Pr | Praseodymium | |||
| 60 | Nd | Neodymium | |||
| 61 | Pm | Promethium | |||
| 62 | Sm | Samarium | |||
| 63 | Eu | Europium | |||
| 64 | Gd | Gadolinium | |||
| 65 | Tb | Terbium | |||
| 66 | Dy | Dysprosium | |||
| 67 | Ho | Holmium | |||
| 68 | Er | Erbium | |||
| 69 | Tm | Thulium | |||
| 70 | Yb | Ytterbium | |||
| 71 | Lu | Lutetium | |||
| 72 | Hf | Hafnium | |||
| 73 | Ta | Tantalum | |||
| 74 | W | Tungsten | |||
| 75 | Re | Rhenium | |||
| 76 | Os | Osmium | |||
| 77 | Ir | Iridium | |||
| 78 | Pt | Platinum | |||
| 79 | Au | Gold | |||
| 80 | Hg | Mercury | |||
| 81 | Tl | Thallium | |||
| 82 | Pb | Lead | |||
| 83 | Bi | Bismuth | |||
| 84 | Po | Polonium | |||
| 85 | At | Astatine | |||
| 86 | Rn | Radon | |||
| 87 | Fr | Francium | |||
| 88 | Ra | Radium | |||
| 89 | Ac | Actinium | |||
| 90 | Th | Thorium | |||
| 91 | Pa | Protactinium | |||
| 92 | U | Uranium | |||
| 93 | Np | Neptunium | |||
| 94 | Pu | Plutonium | |||
| 95 | Am | Americium | |||
| 96 | Cm | Curium | |||
| 97 | Bk | Berkelium | |||
| 98 | Cf | Californium | |||
| 99 | Es | Einsteinium | |||
| 100 | Fm | Fermium | |||
| 101 | Md | Mendelevium | |||
| 102 | No | Nobelium | |||
| 103 | Lr | Lawrencium | |||
| 104 | Rf | Rutherfordium | |||
| 105 | Db | Dubnium | |||
| 106 | Sg | Seaborgium | |||
| 107 | Bh | Bohrium | |||
| 108 | Hs | Hassium | |||
| 109 | Mt | Meitnerium | |||
| 110 | Ds | Darmstadtium | |||
| 111 | Rg | Roentgenium | |||
| 112 | Cn | Copernicium | |||
| 113 | Nh | Nihonium | |||
| 114 | Fl | Flerovium | |||
| 115 | Mc | Moscovium | |||
| 116 | Lv | Livermorium | |||
| 117 | Ts | Tennessine | |||
| 118 | Og | Oganesson |



















































